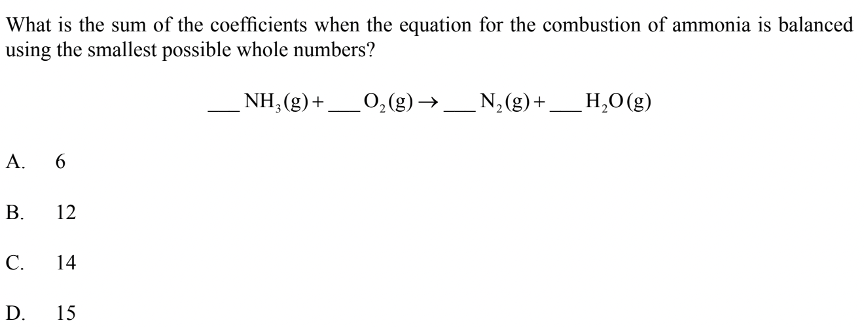IB Chemistry SL Exam Practice Questions
What’s Covered:
- Overview of the IB Chemistry SL Exam
- IB Chemistry SL Exam Questions
- Calculator Suggestions for Chemistry SL
- Final Tips
- How Does the IB Chemistry SL Exam Affect My College Chances?
Chemistry SL is one of the most popular International Baccalaureate Diploma Programme (IBDP) subjects thanks to its great inclusion of lab practices and technical methods of studying. One area most students tend to struggle with in Chemistry, or any IB subject for that matter, are examinations. Exams are the primary way of earning a grade in IB and they can be daunting and stressful, but there’s a workaround to that!
This guide is going to prepare you for the IB Chemistry SL examinations with practice questions, to familiarize yourself with what would otherwise be difficult problems on exam day!
Overview of the IB Chemistry SL Exam
The IB Chemistry SL exam is divided into three individual components called papers, with there being 3 different papers. The three papers are designed to test students on different aspects of the science, and with different styling in the questions too. The kinds of questions, although styled differently, will all still stem from the same coursework provided during the two-year course.
The formatting of the papers is as follows:
- Paper 1: 30 Points (MCQ) / 45 minutes long / Calculator not permitted
- Paper 2: 50 Points / 75 minutes long / Calculator permitted
- Paper 3: 35 points / 60 minutes long / Calculator permitted
A data booklet and periodic table are also always provided for all three exams.
IB Chemistry SL Exam Questions
There really isn’t a better way to study for IB exams than to attempt and solve questions from previous examinations!
The following questions are gathered from a specimen paper. Specimen papers contain questions that will be the rough foundation on which the actual exam papers are created. You can find these papers at your school.
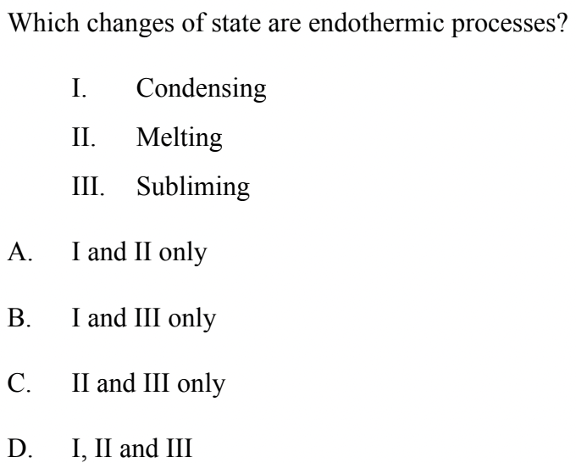
Paper 1 – Question 1
Solution: C
Melting is an endothermic process because the ice absorbs heat which results in a change in temperature. Subliming is also an endothermic process because it requires absorption of heat/thermal energy to convert a solid into a gas. These two processes being endothermic rules out options A and B. The correct answer is C because condensing releases or gives out energy in the process, making it exothermic instead of endothermic.
Paper 1 – Question 2
Solution: D
D is the correct answer here because if we balance the compounds on both sides we get \(4NH_3\), \(3O_2\), \(2N_2\), and \(6H_{2}O\). This is the simplest form in which the equation above can be balanced and true.
Adding these coefficients together gives us 15, which is option D.
Paper 1- Question 3
Solution: C
As we go down a group, the number of energy levels increases, which creates a greater distance between the nucleus and outermost orbital level of electrons. This distance is also referred to as the atomic radius, hence option C.
That’s all for paper 1! It may not seem difficult, but the real challenge is solving those “easy” questions with the tight time limit. Practice your speed with these papers, and that’s the key to achieving a better grade!
Now let’s take a look at some paper 2 questions, which are a little more difficult.
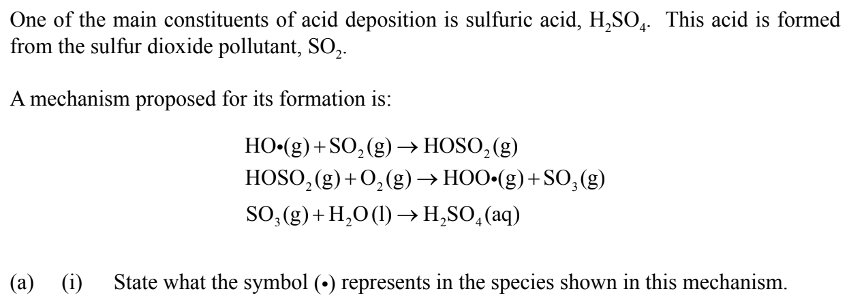
Paper 2 – Question 1 – Part 1
Solution: Radical or Unpaired Electron
This is the way radical or unpaired electrons are denoted in chemistry; it’s important to follow the required nomenclature for maximum points.
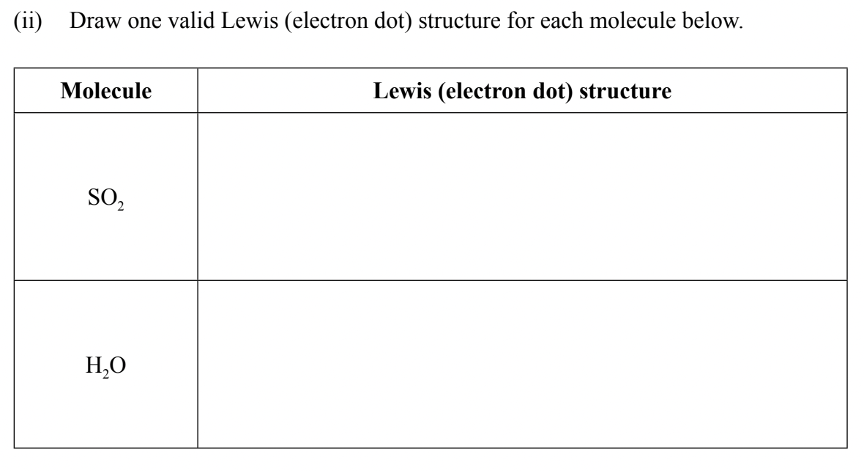
Paper 2 – Question 1 – Part 2
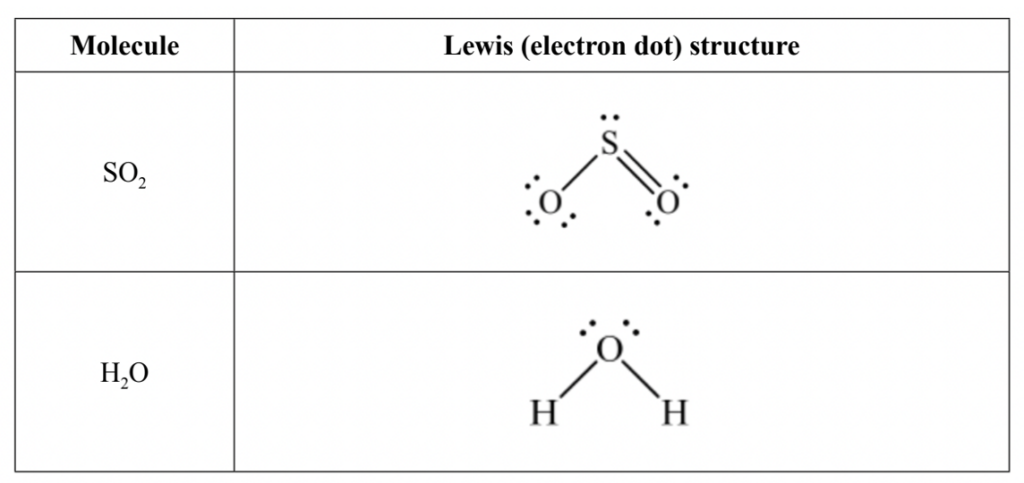
Solution: We can use x’s or dots to represent electron pairs. Lewis structures only use/consider valence electrons (the outermost electrons), to demonstrate how elements bond with each other to form compounds. The lewis structures for the two molecules are as such:
This is correct because for each molecule we have correctly shown the number of valence electrons available in the outermost orbital of the elements, along with which ones are being used to form covalent bonds, as represented with the lines.
Paper 2 – Question 2
Solution: This is because only water is produced as a by-product, which is non-polluting compared to the exhaust fumes of a regular combustion engine.
That wraps up paper 2. The questions are noticeably lengthier and harder than paper 1 and a bulk of the points for the exams come from this paper. Paper 3 questions will be more focused on experimental practices and lab techniques.
Paper 3 is divided into two sections, part A and B. A is general questions about lab technique, but part B is about more specific concepts based on your choosing. When you sign up for IB, you choose a couple extra subjects in the coursework as additives to learn on top of the required amount, these are referred to as options. Options range from numerous concepts but based on which option you selected, your part B of paper 3 will differ. For this exam review, however, we will focus on part A.
Paper 3 – Question 1 – Part 1
Solution:
Thomas forgot to account for water crystallization. This is when water molecules form to create an essential part of the crystal structure for many crystal compounds.
Paper 3 – Question 1 – Part 2
Solution: Two reasons include:
- There is less uncertainty in the volume when using a volumetric flask thanks to functionality towards measurement
- A volumetric flask is also capable of taking changes in volume into account when dissolving substances.
Paper 3 – Question 1 – Part 3
Solution: Thomas can obtain a pure and dry solid product by using either a filter or a centrifuge. Both devices will separate the pure and dry solids from any other substances.
That marks the end of our review for paper 3. This one question actually covers a lot of bases for paper 3 and goes over the most mentioned and tested experimental techniques in IB Chemistry SL. Make sure to reach out to your school for more resources such as past papers and exams to better prepare yourself.
Calculator Suggestions for Chemistry SL
Calculator selection isn’t as important in chemistry as it is in other IB science or STEM subjects, but it is used occasionally for mole calculations and some other things. That being said, a good calculator that runs well and streamlines mathematical processes can only make exam-taking an easier process! The following calculators are just recommendations, but if you find that you’re comfortable with a calculator of your choosing then feel free to use that!
Suggestions:
- Ti84 plus (all models)
- Ti Nspire (non-CAS models) with the 84 faceplate
- Ti83 plus
- Casio CFX 9850 Plus
- Casio FX 9950 Plus
Final Tips
Test taking and exam season are stressful things related to IB, but it doesn’t need to be that way. The following tips may help you out with some exam anxiety, and help you perform as best as you can!
Get Enough Rest
A lot of students cram their studying into the night before the exam. That’s never a good idea for two reasons. Firstly, it’s impossible to retain so much information in such a short period of time. To solve this you should always begin studying as early as you can. Secondly, the lack of sleep/rest before the exam could lead you to be in a state that isn’t best suited for your peak exam performance. Also, resting with less studying the night before could arguably be better than cramming and not catching a wink of sleep!
Practice Your Speed
Paper 1 is considered easy in content, but the amount of time for the paper can make it challenging. To combat this, start practicing paper 1 as early as possible under practical exam-like situations. This means having a time limit on your practice! By practicing in more exam-based situations you can better prepare yourself for the real deal with more realistic expectations!
How Does the IB Chemistry SL Exam Affect My College Chances?
The exam doesn’t have any direct impact on your college admission chances. However, sticking through with exams and showing the determination to continue classes that are considered hard, does speak volumes. This level of effort is appreciated by admissions officers and it’s much rather what they’d scout for instead of scores.
That being said, it can still be really difficult to manage reasonable expectations of what admission into a college looks like for you. Fortunately, CollegeVine’s admissions calculator makes this much easier! This tool uses information ranging from your standardized test scores to your extracurriculars and personal details to calculate your unique chances of getting into a college of your choosing!